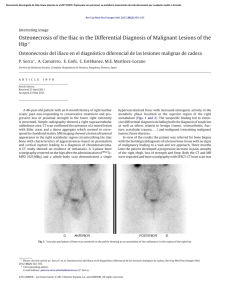
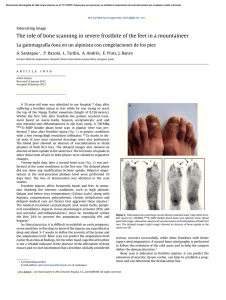
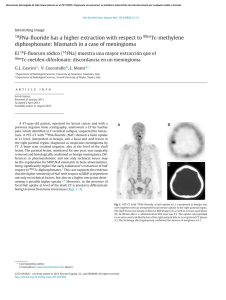
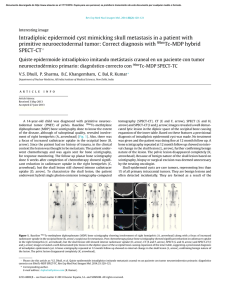
Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Contents lists available at ScienceDirect Journal of Cranio-Maxillo-Facial Surgery journal homepage: www.jcmfs.com Evaluation of success of alveolar cleft bone graft performed at 5 years versus 10 years of age* de ric Bodin a, Bruno Grollemund a, Thomas Bridonneau a, Caroline Dissaux a, *, Fre Isabelle Kauffmann a, b, Jean-François Mattern c, d, Catherine Bruant-Rodier a a Cleft Competence Center, Maxillofacial and Plastic Surgery Department (Head: Catherine Bruant-Rodier, MD, PHD), Strasbourg University Hospital, ^pital, 67091 Strasbourg, France 1 place de l'Ho b ^pital de Hautepierre, Avenue Moli Pediatric Surgery Department, Strasbourg University Hospital, Ho ere, 67200 Strasbourg, France c ^pital de Hautepierre, Avenue Moli Imaging Department, CBCT Department, Strasbourg University Hospital, Ho ere, 67200 Strasbourg, France d Cabinet de radiologie et d'imagerie m edicale de Bischwiller, 13 rue Poincar e, 67240 Bischwiller, France a r t i c l e i n f o a b s t r a c t Article history: Paper received 17 March 2015 Accepted 17 September 2015 Available online 19 October 2015 Background and purpose: Although alveolar cleft bone grafting is the most widely accepted approach, controversies remain on the operative timing. Methods: A consecutive retrospective series of 28 patients who received alveolar bone grafting was examined and divided into 2 groups depending on the age at the time of bone graft. Group A (14 patients) was operated at a mean age of 5.2 years [range, 4e7] and Group B (14 patients) at a mean age of 10 years [range, 8.5e13]. All the children were assessed clinically and by Cone Beam Computed Tomography (CBCT) before bone grafting and 6 months post-operatively. Cleft and bone graft dimensions, volumes were assessed using Osirix v.3.9.2. Residual bone graft coefficient (Bone Graft Volume on 6-months Postoperative CBCT/ Alveolar Cleft Volume) was calculated. Complications, tooth movement or dental agenesis were also reported. Results: The sample was uniform within both groups, considering cleft forms, pre-surgical fistula rate and cleft volume. Residual bone graft coefficient reached 63.3% in Group A and 46.2% in Group B (p ¼ 0.012). Results of residual bone graft are also influenced by tooth eruption through the graft (p ¼ 0.007 in Group A and p ¼ 0.02 in Group B). Conclusions: This 3D analysis highlighted higher success of alveolar bone grafts when children are operated earlier around 5 years. Level of evidence: Therapeutic study. Level III/retrospective comparative study. © 2015 European Association for Cranio-Maxillo-Facial Surgery. Published by Elsevier Ltd. All rights reserved. Keywords: Cleft lip and palate Alveolar bone grafting Treatment outcome measures 1. Introduction In patients with cleft lip and palate, managing residual alveolar cleft is essential to restore maxillary segment union and stability, allow tooth eruption, close alveolar fistula, and provide support to the lip and the nose (Witsenburg, 1985). * Reference institution of the study: Maxillofacial and Plastic Surgery Depart^pital, ment, Cleft Competence Center, Strasbourg University Hospital, 1 place de l'ho 67091 Strasbourg, France. * Corresponding author. Maxillofacial and Plastic Surgery Department, Stras^ pital Civil, 67091 Strasbourg, France. bourg University Hospital, 1 place de l'Ho Tel.: þ33 6 59433615. E-mail addresses: [email protected], [email protected] (C. Dissaux). However, different modalities may be used. Before the 1970s, primary osteoplasty was routinely performed until Robertson (Robertson and Jolleys, 1968) and Rherm (Rherm et al., 1969) showed its adverse developmental effect on maxillary growth. Secondary alveolar bone grafting has since been developed and is now well established following the original work of Boyne and Sands (1972). The bone that is most commonly used is the cancellous iliac bone, but the tibial shaft, mandible, rib, and calvaria may also be used. The procedure is usually performed when the patient is between 9 and 12 years old, in a mixed dentition, before eruption of the canines so that they may erupt through the grafted site. More recently, other authors (Borstlap et al., 1990; Lilja et al., 2000; Talmant et al., 2002) have advocated that alveolar bone http://dx.doi.org/10.1016/j.jcms.2015.09.003 1010-5182/© 2015 European Association for Cranio-Maxillo-Facial Surgery. Published by Elsevier Ltd. All rights reserved. 22 C. Dissaux et al. / Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Table 1 Patient characteristics and distribution of cleft forms. Age at time of alveolar bone graft (years) Number of patients UCLA UCLP BCLP Number of grafts Sex ratio Retro-alveolar fistulas Orthopedic maxillary expansion Group A Group B 5.2 (4; 7) 10 (8.5; 13) 14 2 10 2 (1 only grafted on 1 side) 15 4 F/10 M 3 13 14 2 9 3 (1 only grafted on 1 side) 16 3 F/11 M 3 13 UCLA ¼ unilateral cleft labio-alveolar; UCLP ¼ unilateral cleft lip and palate; BCLP ¼ bilateral cleft lip and palate. grafting be performed before the eruption of maxillary lateral incisors, when the patient is between 4 and 6 years old. The graft allows the lateral incisor to erupt through the grafted bone, and these authors reported better results in terms of residual bone height. The primary aim of this study was to compare the results of bone grafts performed in patients at 5 and 10 years of age. The secondary aim was to show the value of Cone Beam Computed Tomography (CBCT) in bone graft assessment. 2. Material and methods Twenty-eight consecutive pediatric patients received secondary alveolar bone grafts between January 2012 and February 2013 at our institution. Inclusion criteria were as follows: (a) Children had to have undergone operation by the same surgeon; (b) Children had residual alveolar clefts. The same operations were performed during the first year of life (3 months: Millard lip closure without gingivoperiosteoplasty; 6 months: one-stage palatoplasty using VeaueWardill flaps); (c) No patients belonged to a syndrome; (d) No attempt at previous grafting had been done. Patients were divided into two groups. Group A (14 patients) received secondary bone graft at a mean age of 5.2 years (range: 4e7 years); and Group B (14 patients) underwent operation at a mean age of 10 years (range: 8.5e13 years). Patient characteristics and distribution of cleft forms within both groups are presented in Table 1. Fistulas located right behind the maxillary arch, also known as retroalveolar fistulas, were also recorded. In each group, 13 patients required maxillary transversal expansion before grafting. Secondary alveolar bone grafting was performed following previously described principles (Boyne and Sands, 1972; Abyholm et al., 1981; Bergland et al., 1986), and the cancellous bone harvested from the iliac crest was used. Patients were asked to begin to brush their teeth 1 day after surgery and to have a full liquid diet for 10 days and then to eat food that did not need to be chewed for an additional 3 weeks. All children were assessed clinically and by CBCT (QR Newtom 5G, 1 slice/0.15 mm) the day before bone grafting and 6 months after surgery. Tooth movements were recorded on clinical examination and CBCT images. Early and late complications were also reported. 2.1. Data acquisition Preoperative CBCT was used to assess cleft volume and dimensions, whereas postoperative CBCT allowed estimating the residual graft volume. All data recorded in Digital Imaging and Communications in Medicine (DICOM) format were transferred to Osirix v.3.9.2 software. On preoperative CBCT images, the maximal height, maximal width, and maximal length of the cleft were measured using the rulers along the edges. On postoperative CBCT images, the same dimensions were reported for the graft. Maximal graft dimensions are expressed as a percentage of maximal cleft dimensions. Alveolar clefts and bone grafts were delimited on each slice using the drawing tool. The X, Y, and Z planes, as well as scanning information, were used to delineate the measurement areas for the alveolar cleft and the graft. This way, the alveolar cleft volume was measured on transversal areas between two sagittal planes and two coronal planes (an upper area on the nasal floor and a lower area on the tooth cervix). These planes were based on the limit between the bone and the air on the noncleft side. Multiplanar views or a single plane in the full-screen window could be selected to facilitate graft edge location, as well as the corresponding preoperative image superimposition. Collection of these slices was stacked to produce a 3D volume (Fig. 1). The volume of the entire structure was calculated and shown by the system. The residual bone graft coefficient (%) was calculated using the following formula: Bone Graft Volume on CBCT image 6 months post surgery/Alveolar Cleft Volume. Fig. 1. Example of bone graft volume acquisition. The alveolar bone grafts were delimited on each slice using the drawing tool. Transversal areas were superimposed to produce a 3D volume using Osirix v.3.9.2 software. C. Dissaux et al. / Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Table 2 Preoperative alveolar cleft dimensions and volume. Height (cm) Width (cm) Anteroposterior length (cm) Volume (cm3) Group A Group B 1.10 (0.8; 1.5) 1 (0.65; 1.2) 0.9 (0.7; 1.1) 0.965 (0.7; 1.3) 1.22 (0.9; 1.5) 1 (0.7; 1.3) 0.9 (0.7; 1.1) 1.03 (0.7; 1.5) Volumetric measurements were performed twice by one observer in the horizontal dimension on the same day and once by another observer. palate (UCLP), and two patients with total bilateral cleft lip and palate (BCLP; one grafted on only one side), corresponding to a total of 15 bone grafts. Group B included two patients with UCLA, nine with UCLP, and three with BCLP (1 grafted on only one side), corresponding to a total of 16 grafts. Three retroalveolar fistulas were found during the preoperative clinical examination in each group. On preoperative CBCT images, the dimensions of the alveolar cleft in both groups were comparable, as well as the cleft volumes, with respectively 0.965 cm3 (range: 0.7e1.3) in Group A and 1.03 cm3 (range: 0.7e1.5) in Group B (Table 2). 3.2. Postoperative complications 2.2. Statistical analysis A Student t test was used to compare Groups A and B for quantitative values. A P-value of 0.05 or less was considered statistically significant. The impact of dental agenesis and dental eruption was studied using a Fisher exact test. Only one graft exposure was observed 3 weeks after surgery in Group B. No complication of the graft occurred in Group A. No donor site complications (iliac crest) were observed in either group. 3.3. 2D evaluation (Table 3) 2.3. Ethics considerations The study was conducted in accordance with the tenets of the Declaration of Helsinki (2004) and was approved by Strasbourg University Hospital Ethical Commission. 3. Results 3.1. Patient and cleft characteristics The study sample was homogeneous within both groups in terms of cleft form, presurgery fistula rate (Table 1) and cleft volume (Table 2). Group A included two patients with unilateral cleft labio-alveolar (UCLA), 11 patients with total unilateral cleft lip and Table 3 2D evaluation. Max BG width/Max CA width (%) Max BG height/Max CA height (%) Max anteroposterior BG length/ Max anteroposterior CA length (%) 23 Group A Group B P 100 64.3 85.3 94 53.3 78.5 NS 0.14 0.44 Maximal dimensions of the bone graft are expressed as a percentage of maximal alveolar cleft dimensions. No statistically significant differences were revealed in this analysis. Max ¼ maximal; BG ¼ bone graft; CA ¼ alveolar cleft; NS ¼ not significant (no statistically significant difference). In all cases, the maxillary union was obtained with a sufficient bony bridge on the transversal plane (Fig. 2), except in the case of graft exposure in Group B. The mean maximal height of the bone graft reached 64.2% of the maximal height of the alveolar cleft in Group A and 53.3% in Group B. The mean maximal anteroposterior length of the graft reached 85.3% of the maximal anteroposterior length of the alveolar cleft in Group A and 78.5% in Group B. Intergroup differences were not statistically significant. 3.4. 3D evaluation The mean bone graft volume was of 0.6 cm3 (range: 0.36e1.1) in Group A and 0.45 cm3 (range: 0e0.7) in Group B. The residual bone graft coefficient was of 63.3% in Group A and 46.2% in Group B (P ¼ 0.012). The intergroup difference was statistically significant, with a higher residual bone graft in Group A (Fig. 3). 3.5. Dental evaluation In Group A, the assessment performed 6 months after surgery showed that the lateral incisor had erupted through the bone graft in four cases (four of 15). These four cases obtained the best results Fig. 2. Bony bridge observed 6 months after the alveolar bone graft. A. Image obtained one day before surgery. B. Bony bridge observed 6 months after surgery. 24 C. Dissaux et al. / Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Fig. 3. Distribution of the residual bone graft coefficients within both groups. Mean residual bone graft coefficients were statistically different between Groups A and B (p ¼ 0.012). In Group A, most coefficients were greater than 50% (red line), while in Group B, most of them were less than 50%. Residual bone graft coefficient was calculated using the formula: Volume of alveolar bone graft 6 months after surgery/Volume of alveolar cleft. in terms of residual bone graft 6 months after surgery, with a residual bone graft coefficient ranging between 69% and 90% (Fig. 4). In Group B, the eruption of the maxillary canine through the bone graft was observed in four cases (four of 16). These 4 cases also had the best bone graft results, with a coefficient ranging between 51.4% and 83% (Fig. 4). In both groups, the results of the residual bone graft 6 months post surgery were influenced by the tooth eruption through the graft (P ¼ 0.007 in Group A and P ¼ 0.02 in Group B). The influence of dental agenesis on bone graft results was also investigated (Table 4). Lateral incisor agenesis was shown to influence bone graft results only in Group B (P ¼ 0.002). 4. Discussion Since alveolar bone grafting performed in patients between 9 and 12 years old has been established as the “gold standard” technique, many studies have confirmed its success rate (Bergland et al., 1986; Hynes and Earley, 2003; Schultze-Mosgau et al., 2003; Boland et al., 2009). This study focused on grafts performed at an earlier age, before maxillary lateral incisor eruption. This assessment, which strictly compared results of alveolar bone grafts performed at two different ages before canine eruption (the gold standard) or before lateral incisor eruption, was original. Indeed, major studies have evaluated subjects at different ages without individualizing and comparing these two distinct groups. This study was based on a homogeneous population with an equal distribution of cleft forms, fistulas and comparable sex ratio in both groups. The cleft volume calculated based on CBCT data is comparable to cleft volumes reported in the literature assessed using CT scans. Feichtinger et al. (2008) reported a mean cleft volume of 1.2 cm3 (range: 0.7e1.7) in a series of 20 patients at 11 years of age. Compared to CT, the CBCT device (Arai et al., 1999) allows one to perform an assessment using a very low radiation dose and a higher resolution on a limited area. This way, the volume analysis obtained by adding areas is highly accurate because slices are performed every 0.15 mm. The 2D evaluation overestimated results compared to the 3D evaluation. For instance, results relating to the maximal height or width in this study could appear satisfactory in both groups with no statistically significant difference. This is the reason why most 2D evaluations (Newlands, 2000; Matic and Power, 2008; Boland et al., 2009) based on the Bergland scale (Bergland et al., 1986) provide excellent results with Bergland scores I or II (graft height >50% of cleft height). However, based on the sagittal dimension or the 3D evaluation at 6 months, alveolar bone grafts did not seem to be associated with such good results. Van der Meij (2001), Feichtinger (Feichtinger et al., 2008), and Hamada (Hamada et al., 2005) have already pointed out that the 2D evaluation overestimated results compared to 3D evaluation. Feichtinger et al. also showed that graft resorption takes place mostly in the sagittal dimension, although it seems stable on coronal views. As previously reported (Zhang et al., 2012), we also noticed that a significant bone resorption took place preferentially at the nasal floor (Fig. 5), mostly affecting the graft height dimension. The 3D evaluation also allowed detection of a statistically significant difference between groups. Indeed, the assessment of the Fig. 4. Eruption of the lateral incisor (Group A) and canine (Group B) through the alveolar bone graft. Best results in terms of residual bone graft 6 months after surgery were obtained when the tooth erupted through the graft. C. Dissaux et al. / Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Table 4 Impact of dental agenesis on alveolar bone graft outcomes. Residual BG coefficient Lateral incisor agenesis No lateral incisor agenesis P Group A Group B 61.75% 32.5% 63.5% 54.5% NS P ¼ 0.002 BG ¼ bone graft; NS ¼ not significant (no statistically significant difference). 25 in patients who experienced eruption of the cleft-adjacent tooth. As in Lilja et al. (2000), the question of determining the indication of the graft depending on the eruption status of the adjacent tooth should be raised, especially in the case of lateral incisor agenesis. Thus, if we considered Group B (grafts performed at 10 years), an agenesis of the lateral incisor was shown to have a negative impact on bone graft outcome. Although the presence of a larger bone gap was assumed in the case of lateral incisor agenesis, the practice of waiting for canine eruption in this group did not seem to guarantee good outcomes. Finally, the growth and early secondary bone grafting at the age of 5 may be questioned. The impact on the maxillary growth remains unknown but, thanks to improvements in dentofacial orthopedics, the impaired growth did not seem to be more problematic than for grafting performed at the age of 10. 5. Conclusion In conclusion, CBCT is a key imaging modality for the 3D assessment of alveolar bone grafting using a low radiation dose and a high resolution when the analysis is limited to the maxilla area. This 3D analysis showed a higher success rate of bone grafts when the children underwent operation earlier (around 5 years of age). The impact of the adjacent tooth was primordial and directly influenced the success rate of the bone graft regardless of age. The maxilla growth and evolution of the newly formed bony bridge over the years should be monitored and could give rise to another study, especially if the implant placement, in the case of agenesis, is scheduled at the end of the child growth period. Fig. 5. Significant bone resorption at the nasal floor. maximal dimensions provided only an approximation of the results. The accurate estimation of volumes showed better outcomes for the bone grafts performed earlier (mean residual bone graft coefficient of 63% in Group A versus 46% in Group B). Captier et al. (2003) and Borba et al. (2013) pointed out that the age and graft success were correlated, but they did not include grafts performed in patients at such a young age (i.e., 5 years old) in their analysis. The bone graft volume 6 months after surgery and the residual bone graft coefficient found in our study are similar to those reported by Feichtinger and Van der Meij (2001), whose analyses were based on CT scans of grafts performed in patients of 10 years old (Group B; mean graft volume of 0.45 cm3 and coefficient of 46%) (Table 5), and we obtained better outcomes when Group A (grafts performed at a mean age of 5 years) was considered. When the tooth eruption was included in the analysis, we observed a significant impact of the tooth eruption through the bone graft on its stability. Zhang et al. (2012) and Borba et al. (2013) reported that the resorption rate of the graft was significantly lower Table 5 3D evaluation of alveolar bone graft in literature. Study Residual bone graft coefficient Volume of alveolar cleft Volume of alveolar bone graft Van der Meij et al. (2005) 10-year-old patient; CT scans Feichtinger et al. (2008) 11-year-old patient; CT scans 70% UCLP 55% BCLP 50% UCLP 1.1 cm3 0.45 cm3 3D evaluation of the alveolar bone graft found in the literature. These 2 studies of grafts have been performed using CT scans on children aged 10 years. Both studies showed that the 2D evaluation overestimated outcomes compared to the 3D evaluation. Funding The authors declare no source of support for this study. References Abyholm FE, Bergland O, Semb G: Secondary bone grafting of alveolar clefts: a surgical/orthodontic treatment enabling a non-prosthodontic rehabilitation in cleft lip and palate patients. Scand J Plast Reconstr Surg 1(2): 127e140, 1981 Arai Y, Tammisalo E, Iwai K, Hashimoto K, Shinoda K: Development of a compact computed tomographic apparatus for dental use. Dentomaxillofac Radiol 28: 245e248, 1999 Bergland O, Semb G, Abyholm FE: Elimination of the residual alveolar cleft by secondary bone grafting and subsequent orthodontic treatment. Cleft Palate J 23: 175e205, 1986 riostoplasties Boland FX, Drikes S, Persac S, Peron JM, Delcampe P: Gingivope es a une greffe osseuse: e valuation radiologique. Rev Stomatol Chir associe Maxillofac 110: 193e197, 2009 Borba AM, Borges AH, Vilarinho da Silva CS, Borozoski MA, Naclerio-Homen MG, Miloro M: Predictors of complications for alveolar bone graft. Br J Oral Maxillofac Surg 52: 174e178, 2013 Borstlap WA, Heidbuchel KL, Freihofer HPM, Kuijpers-Jagtman AM: Early secondary bone grafting of alveolar cleft defects: a comparison between chin and rib grafts. J Craniomaxillofac Surg 18: 201, 1990 Boyne PJ, Sands NR: Secondary bone grafting of residual alveolar and palatal clefts. J Oral Surg 3: 87e92, 1972 Captier G, Bigorre M, Mattei L, Delestan C, Montoya P: La greffe osseuse secondaire s techniques et indans les fentes labio-maxillo-palatines totales: modalite dications a propos de 62 greffes. Ann Chir Plast Esthet 48: 20e30, 2003 € ck R, K€ Feichtinger M, Zemann W, Mossbo archer H: Three-dimensional evaluation of secondary alveolar bone grafting using a 3D navigation system based on computed tomography: a two-year follow-up. Br J Oral Maxillofac Surg 46: 278e282, 2008 Hamada Y, Kondoch T, Noguchi K, Iino M, Isono H, Ishii H, et al: Application of limited cone beam computed tomography to clinical assessment of alveolar bone grafting: a preliminary report. Cleft Palate Craniofac J 42: 128e137, 2005 Hynes PJ, Earley MJ: Assessment of secondary alveolar bone grafting using a modification of the Bergland grading system. Br J Plast Surg 56: 630e636, 2003 Lilja J, Kalaaji A, Friede H, Elander A: Combined bone grafting and delayed closure of the hard palate in patients with unilateral cleft lip and palate: facilitation of lateral incisor eruption and evaluation of indicators for timing of the procedure. Cleft Palate Craniofac J 37: 98e105, 2000 Matic DB, Power SM: Evaluating the success of gingivoperiosteoplasty versus secondary bone grafting in patients with unilateral clefts. Plast Reconstr Surg 121: 1343e1353, 2008 26 C. Dissaux et al. / Journal of Cranio-Maxillo-Facial Surgery 44 (2016) 21e26 Newlands LC: Secondary alveolar bone grafting in cleft lip and palate patients. Br J Oral Maxillofac Surg 38: 488e491, 2000 Rherm AH, Koberg WR, Koch H: Long term postoperative results of primary and secondary bone grafting in complete clefts of the lips and palate. Cleft Palate J 7: 206e212, 1969 Robertson NRE, Jolleys A: Effects of early bone grafting in complete clefts of the lip and palate. Plast Reconstr Surg 42: 414e420, 1968 Schultze-Mosgau S, Nkenke E, Schlegel AK, Hirschfelder U, Wiltfang J: Analysis of bone resorption after secondary alveolar cleft bone grafts before and after canine eruption in connection with orthodontic gap closure or prosthodontics treatment. J Oral Maxillofac Surg 61: 1245e1248, 2003 Talmant JC, Lumineau JP, Rousteau G: Prise en charge des fentes labio-maxilloquipe du Dr Talmant a Nantes. Ann Chir Plast Esthe t 47: palatines par l'e 116e125, 2002 Van der Meij AJW: Bone volume after secondary bone grafting in unilateral and bilateral clefts determined by computed tomography scans. Oral Surg Oral Med Oral Path 92: 136, 2001 Witsenburg B: The reconstruction of anterior residual bone defects in patients with cleft lip, alveolus and palateda review. J Maxillofac Surg 13: 197e208, 1985 Zhang W, Shen G, Wang X, Yu H, Fan L: Evaluation of alveolar bone grafting using limited cone beam computed tomography. Oral Surg Oral Med Oral Pathol Oral Radiol 113: 542e548, 2012