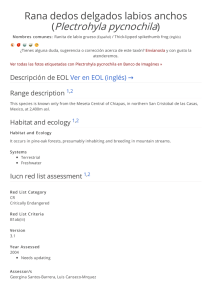
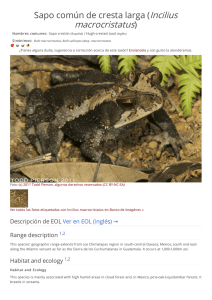
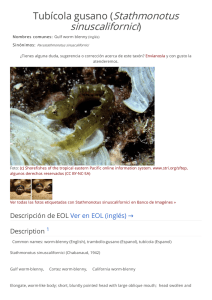
The IUCN Red List of Threatened Species™ ISSN 2307-8235 (online) IUCN 2008: T22697817A132605004 Scope: Global Language: English Spheniscus humboldti, Humboldt Penguin Assessment by: BirdLife International View on www.iucnredlist.org Citation: BirdLife International. 2018. Spheniscus humboldti. The IUCN Red List of Threatened Species 2018: e.T22697817A132605004. http://dx.doi.org/10.2305/IUCN.UK.20182.RLTS.T22697817A132605004.en Copyright: © 2018 International Union for Conservation of Nature and Natural Resources Reproduction of this publication for educational or other non-commercial purposes is authorized without prior written permission from the copyright holder provided the source is fully acknowledged. Reproduction of this publication for resale, reposting or other commercial purposes is prohibited without prior written permission from the copyright holder. For further details see Terms of Use. The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. If you see any errors or have any questions or suggestions on what is shown in this document, please provide us with feedback so that we can correct or extend the information provided. THE IUCN RED LIST OF THREATENED SPECIES™ Taxonomy Kingdom Phylum Class Order Family Animalia Chordata Aves Sphenisciformes Spheniscidae Taxon Name: Spheniscus humboldti Meyen, 1834 Common Name(s): • English: • Spanish: Humboldt Penguin, Peruvian Penguin Pingüino de Humboldt Taxonomic Source(s): SACC. 2005 and updates. A classification of the bird species of South America. Available at: #http://www.museum.lsu.edu/~Remsen/SACCBaseline.htm#. Identification Information: 65 cm. Medium-sized, black-and-white penguin. Black head with white border extending from eye around ear-coverts and chin, and joining on throat. Blackish-grey upperparts. Whitish underparts with black breast-band extending down flanks to thigh. Fleshy-pink base to bill. Juvenile has wholly dark head (greyer on sides and chin) and lacks breast-band. Similar spp. Magellanic Penguin S. magellanicus has broader white stripe on head and has more than one breast-band. Assessment Information Red List Category & Criteria: Vulnerable A2bcde+3bcde+4bcde ver 3.1 Year Published: 2018 Date Assessed: August 9, 2018 Justification: This species has undergone extreme population size fluctuations at major colonies in Chile, but there are considerable uncertainties in the population size and trend. Observed declines in Peruvian colonies and an overall reduction in the number of breeding colonies indicate that there is probably an ongoing, underlying rapid decline in numbers. The species consequently qualifies as Vulnerable. However, if the Chilean population, which comprises the majority of the global population, is found to be stable, the species will warrant downlisting in the future. Previously Published Red List Assessments 2017 – Vulnerable (VU) http://dx.doi.org/10.2305/IUCN.UK.2017-1.RLTS.T22697817A111228184.en 2016 – Vulnerable (VU) http://dx.doi.org/10.2305/IUCN.UK.2016-3.RLTS.T22697817A93641822.en 2013 – Vulnerable (VU) © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 1 http://dx.doi.org/10.2305/IUCN.UK.2013-2.RLTS.T22697817A50410743.en 2012 – Vulnerable (VU) 2010 – Vulnerable (VU) 2008 – Vulnerable (VU) 2005 – Vulnerable (VU) 2004 – Vulnerable (VU) 2000 – Vulnerable (VU) 1994 – Lower Risk/near threatened (LR/nt) 1988 – Threatened (T) Geographic Range Range Description: Spheniscus humboldti occurs along the coastal zone from Isla Foca (5° 12´S) in Peru down to Isla Guafo (43° 32´S) in southern Chile. At least 49 breeding sites have been confirmed between Punta Aguja (5° 47´S) and Isla Metalqui (42° 12´S) in Peru and Chile, respectively (Ayala et al. 2004, Reyes-Arriagada et al. 2009). The global population is estimated at nearly 32,000 mature individuals with key colonies at Punta San Juan (3,160) and Isla Santa Rosa (3,490) in Peru, and Pan de Azúcar (1,600), Chañaral (14,000), Choros (1,860), Tilgo (2,640), and Pajaros Island (1,200) in Chile. A vagrant individual was recorded in Alaska, although it likely was transported by boat (Van Buren and Boersma 2007). Historically, the population suffered a severe decline starting in the mid-1800s due to the extensive guano harvest in Peru and northern Chile, which removed the preferred nesting habitat (Murphy 1936). According to Johnson (1965), the Humboldt Penguin occurred by the “hundreds of thousands” before the guano exploitation started. In the early 1980s, just prior to the 1982-83 El Niño event, the global population was estimated at 16,000-20,000 birds (Hays 1986, Araya and Todd 1988). After this El Niño event, numbers dropped to 5,000-6,000 individuals, but it is uncertain whether this reduction actually represented mortality or dispersal or a combination of both (Hays 1986, Araya and Todd 1988). Evidence from satellite-tracked individuals suggests that part of the population migrate between 6001,000 km northwards during the winter. This has been observed from colonies in northern Chile (Culik and Luna-Jorquera 1997a) and southern Chile (Pütz et al. 2016). Based on band recoveries, Wallace et al. (1999) showed dispersal of penguins from a colony in central Chile up to 600 km to the south and 80 km to the north. Recent geolocation sensor data for Peru indicates post moult movements from breeding individuals at Punta San Juan (15° 22'S) southward up to the Magellan Region (49° 51'S) in Chile (Paredes et al. unpubl. data). Country Occurrence: Native: Chile; Peru Vagrant: Colombia; Ecuador; United States (Alaska) © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 2 Distribution Map Spheniscus humboldti © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 3 Population Counts of moulting birds including mature and immature individuals suggest an average population of c.33,400 ± 2,400 individuals for Chile (Wallace and Araya 2015) and c.10,900 ± 6,900 individuals for Peru (P. McGill pers. comm.). This roughly equates to a global population of 32,000 mature individuals. Trend Justification A current analysis of the population trend reveals high uncertainty in the quality of population numbers taken in the past three generations, with important deficiencies in the coverage of breeding sites and in the methodology employed to count penguins within and between Peru and Chile (Simeone and Cárdenas unpubl.). Most breeding colonies in Peru declined in numbers between 1980 and 2008 (Vianna et al. 2014). In contrast, some colonies in northern Chile showed a positive trend over the same time period; however, the significant population increase in the largest breeding colony at Chañaral Island was attributed to a considerable underestimation of penguin numbers in the past, and thus likely does not represent a real increase in numbers (Mattern et al. 2004, Vianna et al. 2014). Colonies in central Chile showed a stable or negative trend. As a consequence, interpreting the current trend of the global population is problematic and further research is needed. However, considering the lack of evidence for an overall stability or increase in numbers, the current population trend is precautionarily retained as declining. Current Population Trend: Decreasing Habitat and Ecology (see Appendix for additional information) Breeding sites It nests on islands and rocky coastal stretches, using a variety of nest types including guano and dirt burrows, surface nests, vegetation- and rock-covered scrapes, rock crevices, sea caves and under rocks at breakwater walls (Battistini and Paredes 1999, Simeone and Bernal 2000, Paredes and Zavalaga 2001). It apparently prefers to breed on slopes at high elevation sites where guano deposits are available for burrow excavation (Paredes and Zavalaga 2001). Reproductive behaviour Breeding occurs year-round, but has two peaks, in autumn-winter (April through July) and in spring (August through December), with latitudinal shifts in dates between Peru and Chile (Paredes et al. 2002, Simeone et al. 2002, de la Puente et al. 2013). Moult Birds moult mainly during January and February, but moult in juveniles is less synchronous (Simeone et al. 2002, Paredes et al. 2003, de la Puente et al. 2013). Migratory range It is uncertain whether this is a migratory species, but a part of the population migrate after moult (March), with birds from Pan de Azúcar migrating over 600 km (Culik and Luna-Jorquera 1997b) and birds from Puñihuil over 1,000 km (Pütz et al. 2016) northwards from their colonies. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 4 Diet Depending on locality, it feeds on a variety of fish species including Peruvian Anchovy (Engraulis ringens), Araucanian Herring (Strangomera bentincki), Silverside (Odontesthes regia), Common Hake (Merluccius gayi), Inca Scad (Trachurus murphyi), Garfish (Scomberesox saurus scombroides) and South American Pilchard (Sardinops sagax). Diet also includes squid: Dosidiscus gigas and Loligo gahi (Herling et al. 2005). Foraging bahaviour Humboldt Penguins are highly dependent on predictable food resources in coastal waters near the nesting sites (Taylor et al. 2002). During the chick-rearing period, adults forage within 20-35 km around the colony, while incubating birds may reach up to 72 km from the colony (Culik and Luna-Jorquera 1997a, Culik et al. 1998, Chiu et al. 2011). The species typically makes short, shallow dives within 30 m of the surface (Taylor et al. 2002). At Isla Pan de Azúcar, Chile, it was found that maximum dive depth was 53 m. Population health Health surveys conducted on breeding adults from Punta San Juan showed that birds are in good condition. Haematology, plasma chemistries, and plasma mineral levels varied between years. Positive antibody titers for Chlamydophila psittaci (62%), avian adenovirus (7%), paramyxovirus-2 (7%) and Salmonella pullorum (7%) were found (Smith et al. 2008). Sallaberry-Pincheira et al. (2015) detected Haemoproteus sp. in wild ranging Humboldt Penguins at Punta San Juan, but it is unclear whether Haemoproteus sp. sporozoites are able to infect and develop in penguin cells (see Levin et al. 2013, Valkiunas et al. 2014) and, until this has been conclusively demonstrated, it seems unlikely that these parasites pose a significant threat for their conservation (Vanstreels et al. 2016). Pollution Humboldt Penguins at Punta San Juan were evaluated for 55 important trace elements, including Hg [maximum Hg concentrations in serum (0.0056 ± 0.001 µg/g), whole blood (0.297 ± 0.0683 µg/g), and feathers (1.8 μg/g dw)], but at levels generally not considered to cause health impairment. Plasma samples from the same animals were analysed for 31 polychlorinated biphenyls (PCB) and 11 organochlorine (OC) residues using gas chromatography coupled to an ion trap mass spectrometer and for 15 polybrominated diphenyl ethers (PBDE) using gas chromatography high-resolution mass spectrometry. The detection rate for PCBs was 69%, with congeners 105, 118, 180, and 153 most commonly detected (Adkesson unpubl. data). Systems: Terrestrial, Marine Threats (see Appendix for additional information) The Humboldt Current System has alternating blooms and depletions of productivity triggered by El Niño-La Niña dynamics. During El Niño, prey availability is reduced to penguins (Culik et al. 2000, Taylor et al. 2002), inducing nest abandonment and chick mortality (Paredes and Zavalaga 1998, Simeone et al. 2002). However, La Niña conditions improve food availability, producing higher breeding success and chick survival (Simeone et al. 2002). Increased frequency and intensity of El Niño events will likely harm the Humboldt Penguin by reducing its ability to recover fast enough, as has been observed in the Galápagos Penguin Spheniscus mendiculus (Boersma 1998, Vargas et al. 2006). Flooding has been recorded to cause species mortality and a loss of reproductive success in some areas of this species’s range. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 5 The high number of artisanal gillnet fisheries operating within the species range is a considerable concern for the species, and may be of greater impact than large-scale fisheries (Crawford et al. 2017). Industrial fisheries in Peru and Chile exploit the main prey species of penguins (sardines and anchovies). A study by Jahncke et al. (2004) demonstrated that the removal of forage fish is an important pressing threat to several seabirds in the Humboldt Current Ecosystem, that may be hindering their ability to recover to pre-industrial fisheries population levels. Gillnets from artisanal fisheries regularly entangle and kill penguins both in Chile (Simeone et al. 1999, Wallace et al. 1999, Skewgar et al. 2009) and Peru (Majluf et al. 2002, J. Alfaro-Shigueto pers. comm.), and penguins have been shown to be caught as bycatch in large-scale trawl, porse-seine and long-line fisheries throughout their range (Crawford et al. 2017). Simeone et al. (1999) suggest that more penguins die in gill nets during winter, when birds are away from the colonies. In Peru, illegal use of explosives by fishermen has caused penguin mortality (J. Reyes pers. comm.); this also occurs in northern Chile, although it seems to be infrequent (CONAF 2016). Introduced rats (House Rat Rattus rattus and Brown Rat R. norvegicus) predate unattended eggs at several colonies in north and central Chile (Simeone and Luna-Jorquera 2012) and have also been recorded killing chicks in Punta San Juan, Peru (S. Cárdenas-Alayza pers. obs.). Feral dogs Canis familiaris have been reported to kill adults at Pájaro Niño Island in central Chile (Simeone and Bernal 2000) while feral cats Felis catus have been observed on some islands with breeding colonies in Peru. Native Culpeo Lycalopex culpeo have been noted to cause considerable mortality in coastal colonies in Peru, while gulls and vultures are typical egg and chick predators (M. Cardeña pers. comm.). Disturbance impacts may be locally significant for the species. Penguin colonies are frequently visited by tourists and fishermen collecting seafood or seaweeds in northern Chile (Simeone and Schlatter 1998, Ellenberg et al. 2006, CONAF 2016) and Peru. Simeone and Schlatter (1998) reported considerable trampling of nests by unregulated tourists at Puñihuil Islands and by guano harvesters in Peru (P. Majluf pers. comm.). As the species is extremely sensitive to human presence, breeding success is significantly reduced at frequently visited sites (Ellenberg et al. 2006). Historical declines resulted from over-exploitation of guano, which greatly reduced the availability and quality of nesting habitat (Coker 1920, Murphy 1936). Removal of guano reduces the preferred substrate used by penguins to dig burrows (Murphy 1936, Duffy et al. 1984, Paredes and Zavalaga 2001), but guano miners also increase adult and egg mortality through direct harvest, trampling of nests, direct disturbance to breeding sites and by the introduction of alien species such as dogs and rats (Duffy et al. 1984). The coastal and marine area around the major colonies in northern Chile (29-30°S) is currently threatened by the construction of coal-fired power stations (Cárcamo et al. 2011) and large mining proposals (E. Vilaplana in litt. 2018). An industrial mega-port has been approved in the bay close to Punta San Juan, the largest colony for Peru (P. Majluf pers. comm.). In northern Chile, eggs are collected for local consumption and birds are killed for use as fish and crab bait. There is also the potential for oil spills affecting some colonies. In central Chile, two major oil spills occurred in the period 2015-2016, threatening the colony at Cachagua, which contains 800 mature individuals. Over the longer term, the reproductive success of oiled individuals is likely to be impaired and impacts on the marine environment are likely to impact food resources in concert with climate impacts. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 6 Conservation Actions (see Appendix for additional information) Conservation Actions Underway Colonies in Peru and Chile are monitored regularly. In January 2010, the Peruvian government established the Guano System National Reserve (Decreto Supremo 024–2009-MINAM) (Ministerio de Ambiente 2012, http://www.minam.gob.pe/wp-content/uploads/2013/09/decreto_supremo_0242009.pdf). This network of headlands, capes and islands harbours nesting sites of Humboldt Penguins and protects major foraging areas around them. The San Fernando National Reserve (established in July 2011 by the Decreto Supremo 017–2011-MINAM) is also a major site for penguins. Monitoring and removal of rodents has recently begun at Punta San Juan (Cárdenas-Alayza unpubl.). Recently, the Chilean Forest Service eradicated rabbits from Isla Choros in northern Chile, and they have developed an Action Plan (CONAF 2016) for the species aiming to improve its conservation in the country and particularly within the protected area network. Currently, the National Zoo in Santiago (Chile) is successfully developing an ex-situ programme by raising chicks from neglected eggs taken from wild populations. Recently, the Shimonoseki Marine Science Museum (Japan) succeeded in artificially inseminating female penguins from frozen sperm (K. Ueda pers. comm.). Conservation Actions Proposed Currently, population estimates for Peru and Chile are determined by different methods and this prevents comparisons and estimates of the global population. Therefore, a consolidated census methodology for both Peru and Chile should be established. Determine the optimum survey times and methods for assessing the population size of the species in both countries (e.g. define whether censuses of breeding or moulting birds [or both] should be conducted for the species). Quantify the impact of identified threats on distribution, abundance, and breeding success. Identify and quantify the impacts of climate change on population size, distribution, and breeding success. Determine basic life history parameters at strategic colonies along the species's distribution, including juvenile dispersal and survival, breeding success and population size. Identify critical areas for conservation, both inland (breeding) and at sea (foraging), and monitor strategic colonies to detect changes in abundance and how colonies behave during periods of food abundance and scarcity. Determine whether the fluctuations in numbers observed during El Niño are caused by mortality, dispersion or a combination of both. Generate relevant information for industrial fishery management and policy (define catch quotas and fishery bans based on ecosystem parameters) and monitor targeted prey species. Generate a baseline of health parameters across the species's distribution. Assess whether the current Marine Protected Area (MPA) system effectively protects penguins, and establish further MPAs around strategic colonies to secure feeding grounds of penguins, at least during the breeding season. At colonies where MPAs already exist, enforcement should be coupled with management plans and measurable objectives, so that conservation practitioners can monitor the efficiency of MPAs and adapt to changes as necessary. It is urgent to reduce bycatch in gillnets along the entire distribution of the species. The industrial anchovy fishery is a threat to Humboldt Penguins, so total allowable catches should be set based on trophic and oceanographic models that include ecological parameters and a precautionary approach, reducing fishing pressure during El Niño years. Implement a sustainable guano harvest method in order to minimize disturbance at the breeding colonies and better preserve nesting habitat. Continue eradication of invasive species, particularly rats. Develop educational programmes on fish and seabird conservation for adults and children to better understand economic and conservation trades- © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 7 offs with human well-being. Credits Assessor(s): BirdLife International Reviewer(s): Hermes, C. Contributor(s): Daigre, M., Luna-Jorquera, G., Adkesson, M., Alfaro-Shigueto, J., Amaro, L., Bernal, M., Bussalleu, A., Cardeña, M., Colchao, P., Cárdenas, S., Figueroa, J., Flores, M., Garcia Borboroglu , P., Guillermo, E., Jaime, M., Knauf, G., Luyo, P., Majluf, P., McGill, P., Quispe, M., Reyes, J., Roca, M., Schneider, T., Simeone, A., Ueda, K., Valqui, T., Vilaplana, E. & Zavalaga, C. Facilitators(s) and Compiler(s): Benstead, P., Butchart, S., Calvert, R., Capper, D., Clay, R.P., Cárdenas, S., Hatchett, J., Lascelles, B., Martin, R., Moreno, R., Pearmain, L., Simeone, A. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 8 Bibliography Araya, B. and F. S. Todd. 1987. Status of the Humboldt penguin in Chile following the 1982–83 El Niño. Proceedings of the Jean Delacour/IFCB Symposium, Los Angeles : 148–57. Battistini, G.; Paredes, R. 1999. Nesting habits and nest characteristics of Humboldt penguins at Punta San Juan, Peru. Penguin Conservation 12: 12-19. Boersma, P.D. 1998. Population trends of the Galápagos Penguin: impacts of El Niño and La Niña. Condor 100: 245-253. Cárcamo, P. F.; Cortés, M.; Ortega, L.; Squeo, F. A.; Gaymer, C. F. 2011. Crónica de un conflicto anunciado: Tres centrales termoeléctricas a carbón en un hotspot de biodiversidad de importancia mundial. Revista Chilena de Historia Natural 84: 171-180. Chiu, A.; Cárdenas, S.; Cardeña, M.; Bussalleu, A.; Guerrero, P.; Sandoval, F.; Tremblay, Y. 2011. La ruta del pingüino: uso de hábitat marino y patron de atención al nido por el pingüino de Humboldt (Spheniscus humboldti) en Punta San Juan, Perú. Boletín Informativo UNOP 6: 21-27. Coker, R.E. 1920. Peru's Wealth-producing Birds: Vast Riches in the Guano Deposits of Cormorants, Pelicans, and Petrels which Nest on Her Barren, Rainless Coast. Judd & Detweiler, Washington. CONAF. 2016. Plan nacional de conservación pingüino de Humboldt. CONAF Coquimbo. Crawford, R.; Ellenberg, U.; Frere, E.; Hagen, C.; Baird, K.; Brewin, P.; Crofts, S.; Glass, J.; Mattern, T.; Pompert, J. 2017. Tangled and drowned: A global review of penguin bycatch in fisheries. Endangered Species Research 34: 373–396. Culik, B., J. Hennicke, and T. Martin. 2000. Humboldt penguins outmanoeuvring El Niño. Journal of Experimental Biology 203: 2311–22. Culik, B. M.; Luna-Jorquera, G. 1997. Satellite tracking of Humboldt penguins (Spheniscus humboldti) in northern Chile. Marine Biology 128: 547-556. Culik, B. M.; Luna-Jorquera, G. 1997. The Humboldt Penguin: a migratory bird? . Journal of Ornithology 138: 325–330. Culik, B. M.; Luna-Jorquera, G.; Oyarzo, H.; Correa, H. 1998. Humboldt penguins monitored via VHF telemetry. Marine Ecology Progress Series 162: 279-286. De la Puente, S.; Bussalleu, A.; Cardeña, M.; Valdés-Velásquez, A.; Majluf, P.; Simeone, A. 2013. Humboldt Penguin (Spheniscus humboldti). In Penguins. Natural History and Conservation (Garcia Borboroglu, P. and Boersma, P. D., eds.) University of Washington Press, Seattle, Washington, USA: 265283. Duffy, D. C., C. Hays, and M. A. Plengue. 1984. The conservation status of Peruvian seabirds. In Status and Conservation of the World’s Seabirds. ed. J. P. Croxall, P. G. H. Evans, and R. W. Schreiber, 245–59. ICBP Technical Publication No. 2. Cambridge: International Council for Bird Preservation. Ellenberg, U.; Mattern, T.; Seddon, P. J.; Jorquera, G. L. 2006. Physiological and reproductive consequences of human disturbance in Humboldt Penguins: the need for species-specific visitor management. Biological Conservation 133(1): 95-106. Hays, C. 1986. Effects of the 1982-83 El Niño on Humboldt Penguin colonies in Peru. Biological Conservation 36: 169-180. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 9 Herling, C; Culik, B. M.; Hennicke, J. C. 2005. Diet of the Humboldt penguins (Spheniscus humboldti) in northern and southern Chile. Marine Biology 147: 13-25. IUCN. 2018. The IUCN Red List of Threatened Species. Version 2018-2. Available at: www.iucnredlist.org. (Accessed: 15 November 2018). Jahncke, J., Checkley, D.M. and Hunt, G.L. 2004. Trends in carbon flux to seabirds in the Peruvian upwelling system: effects of wind and fisheries on population regulation. Fisheries oceanography 13(3): 208-223. Johnson, A. W. 1965. The birds of Chile and adjacent regions of Argentina, Bolivia and Peru. Platt Establecimientos Gráficos, Buenos Aires. Levin, I.I.; Zwiers, P.; Deem, S.L.; Geest, E.A.; Higashiguchi, J.M.; Iezhova, T.A.; Jimenez-Uzcategui, G.; Kim, D.H.; Morton, J.P.; Perlut, N.G.; Renfrew, R.B.; Sari, E.H.R.; Valkiunas, G.; Parker, P.G. 2013. Multiple lineages of avian malaria parasites (Plasmodium) in the Galapagos Islands and evidence for arrival via migratory birds. Conservation Biology 27: 1366-1377. Majluf, P., E. A. Babcock, J. C. Riveros, M. Arias-Schreiber, and W. Alderete. 2002. Catch and by-catch of sea birds and marine mammals in the small-scale fishery of Punta San Juan, Peru. Conservation Biology 16: 1333–43. Mattern, T.; Ellenberg, R.; Luna-Jorquera, G.; Davis, L.S. 2004. Humboldt Penguin census on Isla Chañaral, Chile: Recent increase or past underestimate of penguin numbers? . Waterbirds 27: 368–376. Murphy, R.C. 1936. Oceanic Birds of South America, 1 and 2. American Museum of Natural History, New York. Norman, JR, 1938. Paredes, R., and C. B. Zavalaga. 1998. Overview of the effects of El Niño 1997–98 on Humboldt penguins and other seabirds at Punta San Juan, Perú. Penguin Conservation 11: 5–7. Paredes, R.; Zavalaga, C. B. 2001. Nesting sites and nest types as important factors for the conservation of Humboldt Penguins (Sphensicus humboldti). Biological Conservation 100: 199-205. Paredes, R.; Zavalaga, C. B.; Battistini, G.; Majluf, P.; McGill, P. 2003. Status of the Humboldt Penguin in Peru, 1999-2000. Waterbirds 26: 129-138. Paredes, R.; Zavalaga, C. B.; Boness, D. J. 2002. Patterns of egg laying and breeding success in Humboldt penguins (Spheniscus humboldti) at Punta San Juan, Peru. Auk 119: 244-250. Pütz, K.; Raya Rey, A.; Hiriart-Bertrand, L.; Simeone, A.; Reyes-Arriagada, R.; Lüthi, B. 2016. Post-moult movements of sympatrically breeding Humboldt and Magellanic penguins in south-central Chile. Global Ecology and Conservation 7: 49-58. Reyes-Arriagada, R., P. Campos-Ellwanger and R. P. Schlatter. 2009. Avifauna de Isla Guafo. Boletín Chileno de Ornitología: 15: 35-43. Sallaberry-Pincheira, N., Gonzalez-Acuna, D., Herrera-Tello, Y., Dantas, G. P., Luna-Jorquera, G., Frere, E., and Vianna, J. A. 2015. Molecular epidemiology of avian malaria in wild breeding colonies of Humboldt and Magellanic penguins in South America. EcoHealth 12: 267-277. Simeone, A. and Luna-Jorquera, G. 2012. Estimating rat predation on Humboldt Penguin colonies in north-central Chile. Journal of Ornithology 153: 1079-1085. Simeone, A., and M. Bernal. 2000. Effects of habitat modification on breeding seabirds: A case study in central Chile. Waterbirds 23: 449–456. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 10 Simeone, A., and R. P. Schlatter. 1998. Threats to a mixed-species colony of Spheniscus penguins in southern Chile. Colonial Waterbirds 21: 418–421. Simeone, A., B. Araya, M. Bernal, E. N. Diebold, K. Grzybowski, M. Michaels, J. A. Tare, R. C. Wallace, and M. J. Willis. 2002. Oceanographic and climatic factors influencing breeding and colony attendance patterns of Humboldt penguins Spheniscus humboldti. Marine Ecology Progress Series 227: 43–50. Simeone, A., M. Bernal, and J. Meza. 1999. Incidental mortality of Humboldt penguins Spheniscus humboldti in gill nets, central Chile. Marine Ornithology 27: 157–61. Skewgar, E., A. Simeone, and P. D. Boersma. 2009. Marine reserve in Chile would benefit penguins and ecotourism. Ocean and Coastal Management 52: 487–491. Smith, K. M., Karesh, W. B., Majluf, P., Paredes, R., Zavalaga, C., Hoogesteijn Reul, A., and Cook, R. A. 2008. Health evaluation of free-ranging Humboldt penguins (Spheniscus humboldti) in Peru. Avian diseases 52: 130-135. Taylor, SS ; Leonard, ML ; Boness, DJ ; Majluf, P. 2002. Foraging by Humboldt penguins (Spheniscus humboldti) during the chick-rearing period: general patterns, sex differences, and recommendations to reduce incidental catches in fishing nets . Canadian Journal Of Zoology-Revue Canadienne De Zoologie 80(4): 700-707. Valkiūnas, G., Palinauskas, V., Ilgūnas, M., Bukauskaitė, D., Dimitrov, D., Bernotienė, R., Zehtindjiev, P., Ilieva, M. and Iezhova, T.A. 2014. Molecular characterization of five widespread avian haemosporidian parasites (Haemosporida), with perspectives on the PCR-based detection of haemosporidians in wildlife. Parasitology research 113(6): 2251-2263. Van Buren, A. N., and P. D. Boersma. 2007. Humboldt penguins (Spheniscus humboldti) in the Northern Hemisphere. Wilson Journal of Ornithology 119(284–288). Vanstreels, R. E. T., Braga, É. M., & Catao-Dias, J. L. 2016. Blood parasites of penguins: a critical review. Parasitology 143: 931. Vargas, F. H., S. Harrison, S. Rea, and D. W. Macdonald. 2006. Biological effects of El Niño on the Galápagos penguin. Biological Conservation . Biological Conservation 127: 107-114. Vianna, J.A., Cortes, M., Ramos, B., Sallaberry-Pincheira, N., González-Acuña, D., Dantas, G.P.M., Morgante, J., Simeone, A. & Luna-Jorquera, G. 2014. Changes in abundance and distribution of Humboldt Penguin Spheniscus humboldti. Marine Ornithology 42: 153–159. Wallace, R.S. and Araya, B. 2015. Humboldt Penguin Spheniscus humboldti population in Chile: counts of moulting birds, February 1999–2008. Marine Ornithology 43: 107–112. Wallace, R. S.; Grzybowski, K.; Diebold, E.; Michaels, M. G.; Tear, J. A.; Willis, M. J. 1999. Movements of Humboldt Penguins from a breeding colony in Chile. Waterbirds 22: 441-444. Citation BirdLife International. 2018. Spheniscus humboldti. The IUCN Red List of Threatened Species 2018: e.T22697817A132605004. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en Disclaimer To make use of this information, please check the Terms of Use. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 11 External Resources For Images and External Links to Additional Information, please see the Red List website. © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 12 Appendix Habitats (http://www.iucnredlist.org/technical-documents/classification-schemes) Habitat Season Suitability Major Importance? 1. Forest -> 1.4. Forest - Temperate Breeding Marginal - 8. Desert -> 8.3. Desert - Cold Resident Suitable Yes 9. Marine Neritic -> 9.1. Marine Neritic - Pelagic Nonbreeding Suitable Yes 9. Marine Neritic -> 9.10. Marine Neritic - Estuaries Nonbreeding Marginal - 10. Marine Oceanic -> 10.1. Marine Oceanic - Epipelagic (0-200m) Nonbreeding Suitable Yes 12. Marine Intertidal -> 12.1. Marine Intertidal - Rocky Shoreline Breeding Suitable No 12. Marine Intertidal -> 12.2. Marine Intertidal - Sandy Shoreline and/or Beaches, Sand Bars, Spits, Etc Nonbreeding Marginal - 12. Marine Intertidal -> 12.3. Marine Intertidal - Shingle and/or Pebble Shoreline and/or Beaches Nonbreeding Suitable Yes 12. Marine Intertidal -> 12.6. Marine Intertidal - Tidepools Nonbreeding Marginal - 13. Marine Coastal/Supratidal -> 13.1. Marine Coastal/Supratidal - Sea Cliffs and Rocky Offshore Islands Resident Suitable Yes 13. Marine Coastal/Supratidal -> 13.2. Marine Coastal/supratidal - Coastal Caves/Karst Resident Marginal - 15. Artificial/Aquatic & Marine -> 15.11. Artificial/Marine - Marine Anthropogenic Structures Resident Marginal - Threats (http://www.iucnredlist.org/technical-documents/classification-schemes) Threat Timing Scope Severity Impact Score 11. Climate change & severe weather -> 11.1. Habitat shifting & alteration Ongoing Majority (5090%) Slow, significant declines Medium impact: 6 Stresses: 1. Ecosystem stresses -> 1.2. Ecosystem degradation 1. Ecosystem stresses -> 1.3. Indirect ecosystem effects 2. Species Stresses -> 2.3. Indirect species effects -> 2.3.7. Reduced reproductive success Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.1. Species mortality 2. Species Stresses -> 2.3. Indirect species effects -> 2.3.7. Reduced reproductive success 11. Climate change & severe weather -> 11.4. Storms & flooding © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en Causing/could cause fluctuations Low impact: 5 13 3. Energy production & mining -> 3.2. Mining & quarrying 5. Biological resource use -> 5.1. Hunting & trapping terrestrial animals -> 5.1.1. Intentional use (species is the target) 5. Biological resource use -> 5.4. Fishing & harvesting aquatic resources -> 5.4.3. Unintentional effects: (subsistence/small scale) [harvest] 5. Biological resource use -> 5.4. Fishing & harvesting aquatic resources -> 5.4.4. Unintentional effects: (large scale) [harvest] 6. Human intrusions & disturbance -> 6.1. Recreational activities 8. Invasive and other problematic species, genes & diseases -> 8.1. Invasive non-native/alien species/diseases -> 8.1.2. Named species (Felis catus) 8. Invasive and other problematic species, genes & diseases -> 8.1. Invasive non-native/alien species/diseases -> 8.1.2. Named species (Rattus rattus) 8. Invasive and other problematic species, genes & diseases -> 8.2. Problematic native species/diseases -> 8.2.2. Named species (Lycalopex culpaeus) 9. Pollution -> 9.2. Industrial & military effluents -> 9.2.1. Oil spills Ongoing Minority (50%) Slow, significant declines Stresses: 1. Ecosystem stresses -> 1.1. Ecosystem conversion 1. Ecosystem stresses -> 1.2. Ecosystem degradation 2. Species Stresses -> 2.2. Species disturbance Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.1. Species mortality Ongoing Majority (5090%) Stresses: 1. Ecosystem stresses -> 1.3. Indirect ecosystem effects 2. Species Stresses -> 2.1. Species mortality Ongoing Majority (5090%) Stresses: 1. Ecosystem stresses -> 1.3. Indirect ecosystem effects 2. Species Stresses -> 2.1. Species mortality Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.2. Species disturbance Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.1. Species mortality Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.3. Indirect species effects -> 2.3.7. Reduced reproductive success Ongoing Minority (50%) Stresses: 2. Species Stresses -> 2.1. Species mortality Ongoing Minority (50%) Stresses: 1. Ecosystem stresses -> 1.2. Ecosystem degradation 2. Species Stresses -> 2.1. Species mortality 2. Species Stresses -> 2.3. Indirect species effects -> 2.3.7. Reduced reproductive success Slow, significant declines Slow, significant declines Negligible declines Slow, significant declines Slow, significant declines Slow, significant declines Slow, significant declines Slow, significant declines Low impact: 5 Low impact: 5 Medium impact: 6 Low impact: 5 Low impact: 5 Low impact: 5 Low impact: 5 Low impact: 5 Low impact: 5 Conservation Actions in Place (http://www.iucnredlist.org/technical-documents/classification-schemes) Conservation Actions in Place In-Place Research, Monitoring and Planning © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 14 Conservation Actions in Place Action Recovery plan: No Systematic monitoring scheme: Yes In-Place Land/Water Protection and Management Conservation sites identified: Yes, over part of range Occur in at least one PA: Yes Invasive species control or prevention: Yes In-Place Species Management Successfully reintroduced or introduced beningly: No Subject to ex-situ conservation: Yes In-Place Education Subject to recent education and awareness programmes: No Included in international legislation: Yes Subject to any international management/trade controls: Yes Conservation Actions Needed (http://www.iucnredlist.org/technical-documents/classification-schemes) Conservation Actions Needed 1. Land/water protection -> 1.1. Site/area protection 2. Land/water management -> 2.1. Site/area management 2. Land/water management -> 2.2. Invasive/problematic species control 3. Species management -> 3.1. Species management -> 3.1.1. Harvest management 4. Education & awareness -> 4.3. Awareness & communications 5. Law & policy -> 5.2. Policies and regulations Research Needed (http://www.iucnredlist.org/technical-documents/classification-schemes) Research Needed 1. Research -> 1.2. Population size, distribution & trends 1. Research -> 1.3. Life history & ecology 1. Research -> 1.5. Threats 3. Monitoring -> 3.1. Population trends © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 15 Research Needed 0. Root -> 4. Other Additional Data Fields Distribution Continuing decline in area of occupancy (AOO): Unknown Extreme fluctuations in area of occupancy (AOO): Unknown Estimated extent of occurrence (EOO) (km²): 2230000 Continuing decline in extent of occurrence (EOO): Unknown Extreme fluctuations in extent of occurrence (EOO): Unknown Number of Locations: 20 Continuing decline in number of locations: No Extreme fluctuations in the number of locations: No Upper elevation limit (m): 80 Population Number of mature individuals: 32000 Continuing decline of mature individuals: Yes Extreme fluctuations: Yes Population severely fragmented: No No. of subpopulations: 2-100 Continuing decline in subpopulations: Yes Extreme fluctuations in subpopulations: Yes All individuals in one subpopulation: No Habitats and Ecology Continuing decline in area, extent and/or quality of habitat: Yes Generation Length (years): 10.6 Movement patterns: Full Migrant Congregatory: Congregatory (and dispersive) © The IUCN Red List of Threatened Species: Spheniscus humboldti – published in 2018. http://dx.doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22697817A132605004.en 16 The IUCN Red List Partnership The IUCN Red List of Threatened Species™ is produced and managed by the IUCN Global Species Programme, the IUCN Species Survival Commission (SSC) and The IUCN Red List Partnership. The IUCN Red List Partners are: Arizona State University; BirdLife International; Botanic Gardens Conservation International; Conservation International; NatureServe; Royal Botanic Gardens, Kew; Sapienza University of Rome; Texas A&M University; and Zoological Society of London. THE IUCN RED LIST OF THREATENED SPECIES™